pH
pH is the unit for measuring how acidic or basic a substance is.
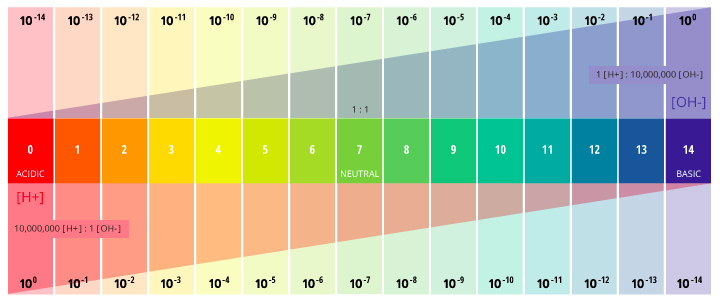
pH is actually stands for "the power of Hydrogen" because it is actually a measure of the relative H+ and OH- concentrations.
pH is measured on a scale from 1-14
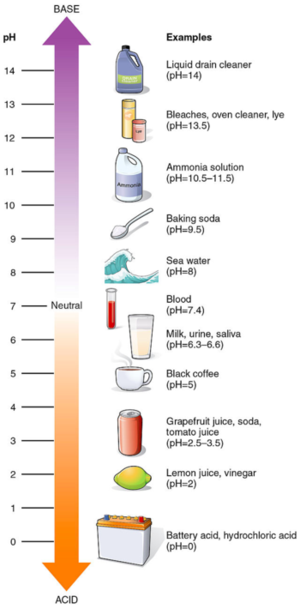
The lower the pH the more acidic a solution is
The higher the pH the more basic a solution is
pH 7 is considered neutral because it has equal concentration of H+ and OH- ions
A strong acid releases more H+ ions and is therefore more acidic and has a lower pH. Increasing the concentration of an acid also increases the concentration of H+ ions making the solution more acidic / lowering the pH.
A strong base releases more OH- ions and is therefore more basic and has a higher pH. Increasing the concentration of a base also increases the concentration of OH- ions making the solution more basic / increasing the pH.
pH is a measure of the relative H+ and OH+ ion concentrations
Low pH = high H+ : low OH-
Neutral pH = equal H+ : OH-
High pH = low H+ : high OH-